PopVax And The Case For Indian Biotech
Biotech is set to explode, and India has a chance to be a major player. We dive into how a Hyderabad startup is leading this charge by marrying computational techniques with rapid experimentation.
I. 🐕 The Shiba Inu Effect
A butterfly shiba inu flaps it wings, launching a biotech tornado across the world
Every great biotech story has to start somewhere. A disillusioned PhD. A forgotten Soviet paper. A stubborn lab rat that just wouldn’t die.
But this one begins with a dog. And not just any dog, but with this ridiculously over-financialized shiba inu.
In August 2020, in the throes of the first global pandemic of our generation, an anonymous crypto entrepreneur named Ryoshi launched a Dogecoin knock-off called Shiba Inu (or SHIB, for short).
In a gimmick meant to lend his creation some instant credibility, Ryoshi airdropped half the total supply of SHIB to a man he reverently addressed as… checks notes… The Woofmeister. He was referring to Vitalik Buterin, the creator of Ethereum and a spiritual compass to the crypto faithful.
Vitalik, at first oblivious to this bizarre act of homage, awoke in May 2021 to a flood of messages. The market cap of SHIB had ballooned overnight. He was now the accidental custodian of some $10 billion US dollars worth of copycat canine currency.
If that last sentence gave you pause, congratulations. You’re still sensitive to absurdity. But remember, this was the season of GameStop rallies, eight-figure NFT sales, and negative interest rates. Financial absurdity was the new normal.
Faced with this surreal windfall, Vitalik responded with rare grace. First, he burned 90% of his SHIB tokens to reduce the circulating supply and stabilize the asset. Then, he donated the rest — worth over $1 billion — to the India COVID Relief Fund, led by Sandeep Nailwal, co-founder of the Polygon blockchain (we’ve written about Polygon’s remarkable origins here).
But that wasn’t quite the end. Recognizing that the Fund was focused on urgent near-term logistics such as sourcing oxygen cylinders and ventilators, Vitalik later reclaimed $100 million of the donation to create a new philanthropic vehicle called Balvi. Its mission: to fund long-horizon, breakthrough science in the fields of vaccine research, air purification, and pandemic resilience. As it turns out, they wouldn’t have to go far to find what they were looking for.
In a stroke of serendipity, Balvi found PopVax: a nascent Hyderabad biotech with the technical chops to reimagine vaccines and the moral clarity to deploy them for the benefit of all humanity. This was exactly what they were looking for. There was only one wrinkle — the founding team had no biology experience whatsoever.
For PopVax, the appearance of Balvi represented an equally unexpected lifeline. After months of unsuccessfully pitching every VC, philanthropy, government fund, and pharma treasury in sight, the company was running out of both time and funding. They desperately needed a visionary backer with the patience to take long-term bets and the courage to look beyond appearances.
Predictably, that idyllic benefactor came in the form of a life sciences fund vehicle dispensing cartoon dog money.
Neither side had quite imagined the other, yet here they were: Balvi, looking to fund breakthrough science for humanity; and PopVax, trying to build exactly that. The result was a $15 million contract1 that was so improbable yet perfectly aligned that it could only signify capital finding its destined purpose.
And that is how a strain of viral dog money, laundered through the internet’s most earnest idealist, found its way to the doorstep of a renegade biotech in Hyderabad.
Now, this unlikely team is reshaping how we think about India’s ability to innovate at the frontier of biology.
This is the story of PopVax — and of the tools, timelines, and tectonic shifts that might just signal a new era for Indian biotech.
Hello and welcome back to Tigerfeathers, your favourite source of deeply-researched yet frustratingly infrequent stories from the world of Indian tech.
Long time no see. There are officially more than 20,000 of us here now, which is the also the number of times I refresh the Premier Leaguer transfer rumours page during the summer. In all seriousness, we are humbled and grateful that so many of you have enjoyed reading our work. We’ve got some epic pieces in store for 2025, starting with today.
This week’s story is particularly exciting because it allows us to do two things we love: i) explain new science that is blowing our minds and ii) shine a spotlight on an inspirational Indian company innovating at the frontier of that science.
Here are just a few of the things you’ll learn in this piece:
How AI is reshaping our ability to understand and design biology
Why mRNA is more than just a COVID-era blip
What building an mRNA therapy looks like, from start to finish
Why India has a tangible advantage in the biotech arena
So let’s get into it, beginning with a word from our sponsor.
This edition of Tigerfeathers is presented in partnership with… Finarkein Analytics.
Finarkein Analytics, backed by Nexus Venture Partners, is a leading player in India's open finance and Digital Public Infrastructure ecosystem. They are trusted by some of the largest banks and NBFCs in India to power mission-critical workflows in lending, wealth, and insurance. If you want to unlock the power of India’s exciting new Account Aggregator framework, reach out to hello@finarkein.com to understand how they can help you transform your business using this revolution in financial infrastructure.
If you're interested in sponsoring a future edition of Tigerfeathers, get in touch with us on Twitter/LinkedIn or by replying to this email. With that, let’s get to it.
II. 🌏 A New World Order
Breaking free of our imaginary biotech chains
At first glance, PopVax’s claims border on the fantastical.
The company says it has developed an influenza vaccine that is five times better at eliciting an immune response in mice than the current best-in-class shot from Sanofi, a blockbuster product that earns the French pharma giant $3 billion every year.
Thanks to their jab’s potential to protect patients across mutating strains of the virus, PopVax has also been awarded a $2 million dollar prize from the US Biomedical Advanced Research and Development Authority (BARDA). And the flu isn’t their only target.
The team is also developing promising candidates for COVID-19, hepatitis C, adult tuberculosis, and even cancer immunotherapy. Cancer, the emperor of all maladies, needs no introduction. But Hepatitis C, though less visible, affects over 50 million people worldwide and still has no vaccine today.
Any single one of these efforts would be impressive. But PopVax has achieved all of this without raising a single rupee in equity funding. And not for lack of trying, either.
Why? Because these claims — especially coming from an Indian startup — defy expectation.
Even PopVax’s founder, Soham Sankaran, acknowledges how improbable it sounds. He says he has gotten used to the raised eyebrows. “For me to publicly claim these numbers,” he told me, “must either mean that I’m stupid — since I’ll surely be found out in the future if I’m lying — or that I’m right.”
His confidence isn’t without caveat: the flu vaccine data comes from preclinical trials in mice. While promising, Soham knows it is not yet definitive. It still needs to be tested in humans. Nevertheless, the ambition is clear, and so is the intent: to meet, and exceed, the highest bar of scientific scrutiny.
The deeper reason people hesitate to believe Popvax’s claims isn’t about animal models or experiment design — it’s about where the data is coming from. Why? Because in biotech, India isn’t expected to innovate. We manufacture. We scale. We copy. But we don’t discover or invent.
Our pharmaceutical industry is the world’s largest supplier of generics, but our R&D spend as a percentage of sales is among the lowest in the world — around 6%, compared to 15–20% for global pharma majors. We’re excellent at process optimization and efficient production of known molecules and biosimilar drugs, but when it comes to novel science, we’ve trained ourselves to defer.
That instinct — to doubt anything frontier-level coming from India — is so deeply ingrained that when groundbreaking data does emerge from an Indian lab, many assume it must be a fluke. Or worse, fraud.
But here’s the thing… a decade ago, people said the exact same thing about China.
And China is now the ascendant force in global biotech. Chinese companies originate more new molecular entities than all of Europe does, make up more than 25% of all new human clinical trial starts, and take billions of dollars in partnerships and capital from the same pharma giants that once dismissed them.
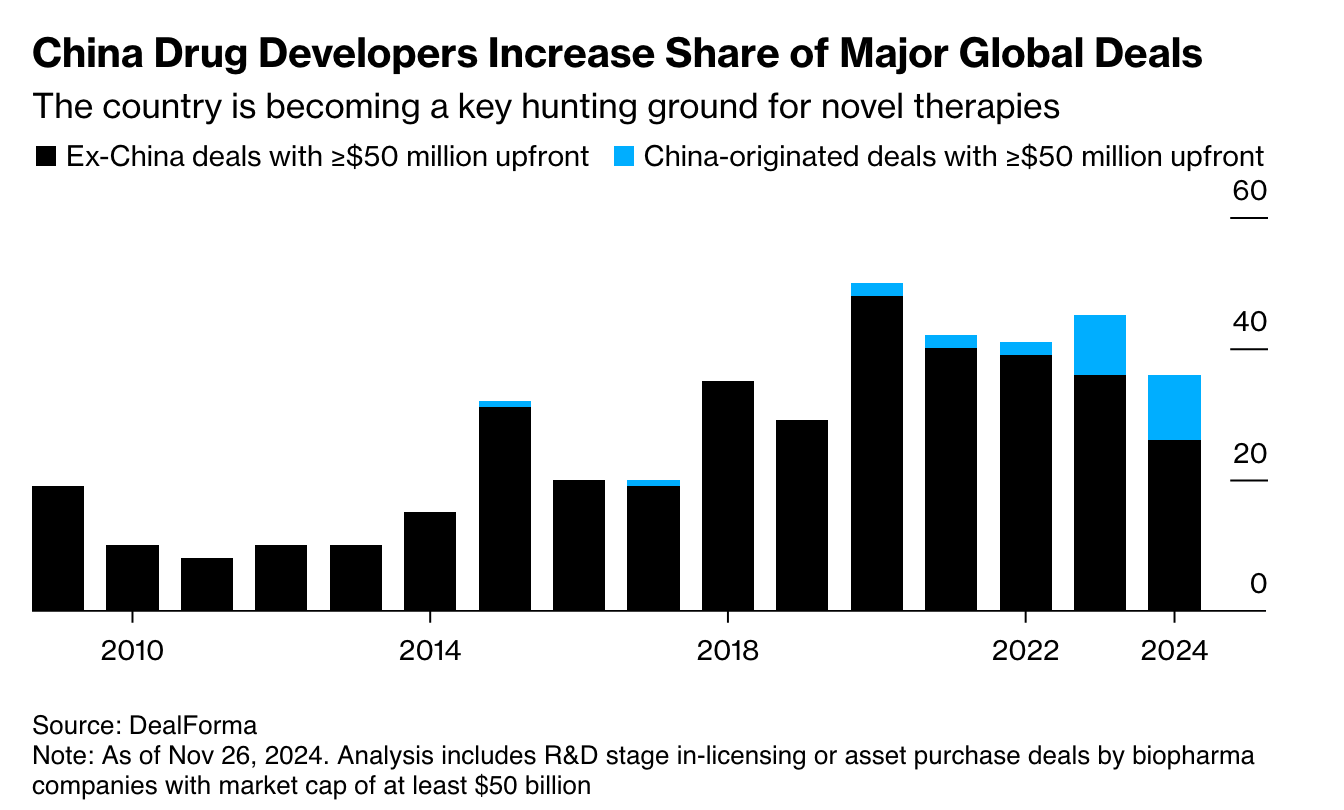
There’s no reason India can’t follow a similar path. The raw inputs exist: talent, capital, demand, and a deep biomedical tradition. What we’ve lacked is belief.
The metaphor that comes to mind is that of an elephant raised in captivity. Once bound by tethers that were too strong to break in its juvenile state, the grown elephant no longer tests its restraints even though it is mighty enough to snap them easily. It has ceased to be an issue of capability, it is now only a matter of conditioning.
Popvax wants to be the whisper that reminds the elephant to push. Although it doesn’t look like a traditional biotech firm from a renowned innovation hotbed, it’s doing world-class science anyway.
That’s what makes it important.
Because this isn’t just about one vaccine, or even one company. It’s about a changing reality: that India, and other emerging nations, can build at the frontier of biology — not someday, but now.
This clarion call is well captured by this manifesto-like passage on Popvax’s homepage.
When we started this company, we were told by many international institutions and investors, as well as many Indian pharmaceutical executives, that we did not have the capability to do mRNA work of any kind in India. We have proved them wrong. We were told that Indians cannot be at the forefront of R&D on a new pharmaceutical platform, that we lack the talent and the rigour and the commitment. We have proved them wrong as well. We are now told that it is folly to imagine that we can leapfrog Merck and Pfizer and GSK, with their hundreds of billions of dollars and decades of dominance. We will prove them all wrong. Hyderabad will be the new global capital of the life sciences, and there will be a new dawn of pharmaceutical R&D across the rising world, from India to Nigeria to Thailand to Bangladesh.
For too long, the developing world has been cast as only a factory floor of global pharma that produces what others design. But PopVax offers a different future: one where countries like India stand shoulder-to-shoulder with the West, inventing and scaling new medicines not just for themselves, but for the entire world.

III. 👨🏾💻 An Unlikely Protagonist
The story of PopVax’ founding
A natural response would be to ask why.
Why did this mission – to rebuild India’s biomedical capability from the ground up – begin not with a pharma CEO or tenured academic, but rather a self-described ‘lapsed computer science researcher’?
Why did Soham Sankaran, at the time leading a Y Combinator-backed company, walk away from his startup, turn away millions in venture capital, and throw himself into a new field he knew nothing about?
The answer begins with a number: six million. That’s how many people are estimated to have died during the COVID-19 pandemic — the majority of them in the Global South, and an overwhelming amount in India.

In a brilliant 2023 essay titled “Three Meetings and Six Million Funerals,” Soham describes this figure not only as a death toll, but as a kind of moral indictment — a number that reveals the structural indifference of global pharma majors, the slowness of public science, and the failure of the developing world to build its own defences.
This realization first struck Soham in 2021, when he found himself locked down in Pune as the newly-evolved Delta variant of COVID-19 ravaged India. Although the world’s largest vaccine manufacturer — the Serum Institute of India — was only a stone’s throw away from him, he could see that our national capability to respond to the pandemic was a world away.
While we had the ability to make many doses of the jab, we simply didn’t have the technology to keep up pace with the rapid mutations of the virus. At the same time, American labs were able to adapt their vaccines to new variants very quickly using mRNA technology. This new technique offered a lot of promise, but India had no equivalent response. No research programs. No national push. No appetite to explore the frontier.
Understandably, the countries possessing these advanced mRNA platforms prioritized their own populations. But in India, as our people ran out of both tears and crematory firewood, our complacency towards innovation was proving fatal. In that moment, Soham saw the price of technological dependence and the urgent need for sovereign scientific capability.
The solutions to the public health crises facing us will not be found in Boston, Geneva, Washington, or Paris, they will be found in Hyderabad and Cape Town and Rio and Bangkok. — popvax.com
The more he learned about mRNA, the more Soham became convinced that it represented a new paradigm for biology. Moreover, the relative nascency of the technology meant that no countries or companies had an unassailable lead when it came to the science and manufacturing. On the contrary, India actually had a few competitive advantages compared to other nations. And bringing those benefits home would give the country the ability to rapidly respond to new viral threats and safeguard its population.
So Soham decided that things would have to change. Not with a speech, but with a lab. And since he didn’t see anyone else stepping up, he resolved to do it himself.
This foray into the field of biotechnology and immunology was an unlikely pivot; Soham’s own parents were, in his words, ‘vaccine-skeptical homeopaths’. But then again, he had always been a maverick and a builder.
Growing up in the beachside suburb of Juhu in Mumbai, Soham spent most of his free time tinkering with technology. After a long day at the Jamnabai Narsee school, you could often find Soham building small robots or writing software at home.
This natural affinity for programming would see him eventually make his way to Yale for an undergraduate degree in computer science, followed by a move to Cornell in pursuit of a PhD in robotics.
But academia couldn’t hold Soham for long. A year into his PhD, he dropped out to launch Pashi, the YC-backed startup that automated and optimized factory production lines.
When the pandemic interrupted his YC batch, Soham moved the company to India. After a few months of living in automotive plants in Pune, Soham was able to deploy his solution in the factories of Indian manufacturing stalwarts like Bajaj Auto. He also started to receive term sheets worth millions of dollars for Pashi.
By all accounts, Pashi was on an exciting path towards something big. But when the devastation from the pandemic became apparent, everything else began to feel small for Soham.
Although he was in Pune to deploy Pashi’s technology at the Bajaj Auto plant, he couldn’t stop thinking about pandemic resilience. He became consumed with the public health problem at hand and threw himself into understanding everything he could about viruses and immunology.
It quickly became clear to him that mRNA technology was the future.
And thanks to a slate of technological advances that we will soon scrutinise, the barriers to entry in this field were rapidly collapsing. You no longer needed tens of millions of dollars to build a vaccine company, you just needed conviction, scientific literacy, and a place to test.
And India — with its pharma manufacturing heritage, huge disease burden, and abundant technical talent — still had a shot at owning this new frontier. But it wasn’t going to happen by default. So Soham apologized to his vaunted customers and shut down Pashi, turned down the term sheets he had been offered, and dove headfirst into bio.
At first, he took a philanthropic angle; he floated the idea of a non-profit that would unite Indian scientific talent and manufacturing companies in building mRNA capability. Although he succeeded in generating excitement amongst Indian diaspora scientists who wished to support this endeavour, Soham had a harder time overcoming the skepticism of pharma CEOs and charitable organizations. Some of them appreciated the intent, but none wrote checks.
“Everyone agreed that we needed to build up this capability eventually, but nobody really believed that it was something that could actually be done in the present.”
So Soham decided to start a for-profit and just do it himself.
He taught himself molecular biology the same way he’d learned software: from scratch. Simultaneously, he started reaching out to global organizations that had an explicit mandate to support vaccine development and research. Starting as someone with… well, basically no experience in this domain whatsoever, Soham initially had a hard time converting these backers.
But as he persevered and grew his knowledge of the field, he began to make a compelling case that he could design effective vaccines leveraging his skills as a computer scientist.
Eventually, his persistence bore fruit. A contact from the Gates Foundation decided to take a gamble on him. Though his bosses in Seattle weren’t entirely convinced, this intrepid program officer prevailed on them to award a $100K contract to the newborn PopVax for early R&D — through the Foundation’s ‘Public Awareness’ budget, no less!
While $100K wasn’t a lot of money for a biotech business, it was enough to run the first experiments. From that seed, the company grew.
Today, PopVax has an 80-member team and operates a full-stack, vertically integrated platform for mRNA vaccine development. They’ve built core capabilities in computational protein design, in vitro transcription, and even their own lipid nanoparticle (LNP) delivery system — all of which we’ll demystify shortly.
And while their earliest funding came from unlikely sources— a marketing budget at the Gates Foundation and a meme coin fortune from Vitalik — PopVax’s science has since earned the respect of institutions not known for sentimentality. The Gates Foundation followed up their initial $100K with a million dollar contract, this time from its actual science budget!
The U.S. government joined in, too: in addition to the $2M prize from BARDA, PopVax was also able to secure the support of the National Institute of Health. The company’s COVID-19 vaccine candidate is now headed to a Phase I human trial in America, fully sponsored by the National Institute of Allergy and Infectious Diseases.
In short, the outsiders have been invited in. Not because of charm or credentials, but because of scientific rigour and hard evidence.
What I find especially compelling about PopVax, in addition to their ambition and execution, is their discernible ethos. Yes, they want to build blockbuster products for global markets, but their deeper goal is to ensure that the tools of modern medicine reach India and the developing world. Not as charity, but as capability.
“We do not need saving,” the company declares on its homepage. “We will do it ourselves.”
This is what a mission-driven organization looks like: guided not by market maps, but by a visceral moral urgency. This business had few precedents to follow and arguably no right to try, either.
And now that it exists, it raises a new question — not about India, but about the state of biology itself:
Why now? Why is it possible, in 2025, for a roboticist with no training or backing to build a world-class biotech company from scratch? What are the tools, technologies, and trends that enable this?
These are the questions that we’ll answer next.
But fair warning, the following section is information-dense. So to prepare you for this journey of learning and discovery, a visual message of motivation.
IV. 💊 A New Era Of Biology, Explained
Understanding the unlocks that enable companies like Popvax, from sequence to shot
A generation ago, building a biotech company required a PhD, a decade, and a nine-figure budget just to get started. Today, someone like Soham Sankaran can teach himself molecular biology, build a vaccine company from scratch, and literally get his programs run by the U.S. government.
So what changed?
Over the past two decades, biology has moved further along the spectrum from mystery to machinery. We now have the tools to read, write, and run the genetic instructions that underpin life. That doesn’t mean biology has become software — it’s still unpredictable, nonlinear, and deeply complex — but it’s become programmable enough for small teams to do things that once required entire institutions.
Rather than explain this shift in the abstract, let’s get into the technical details and how they work in practice. By walking through how Popvax builds a vaccine from start to finish, we can learn how decades of research and innovation are converging to create the conditions for a golden age of biology.
📚 Step 0 - A Crash Course In Vaccines & Proteins
Before getting into the intricacies of vaccine development, we first need to understand what Popvax is actually trying to do.
The goal of Popvax’s vaccine programs is to train the immune system to recognize the shape of a particular protein that it hasn’t seen yet, but might encounter in the future. If the body knows what that shape looks like ahead of time, it will know how to respond appropriately the next time it detects it. That, at its core, is what a vaccine is: a dress rehearsal to get the immune system ready for the real show.
But why a protein?
Because proteins are the machinery of life. Nearly everything your body does — or that happens to it — is mediated by proteins. They carry oxygen, contract muscles, digest food, form skin, and fight infections. If something is going wrong in your body, it’s probably because you either have too much of some protein or not enough of some protein. These complex molecules are not the supporting cast of biology; they are the lead actors.
💪🏽 So what are proteins, anyway?
They are long chains of amino acids — molecules built from carbon, hydrogen, oxygen, nitrogen, and sulfur — that fold into precise three-dimensional shapes. It’s worth emphasizing here that shapes are not incidental to biology; they are the very basis of function. The shape of a molecule is its most important feature.
Depending on what kind of shape it folds into, a protein might pump ions across a membrane, slice up other molecules, bind to a virus, or form fingernails. And the shape of a protein is in turn dictated by the sequence of amino acids that comprise it.
Each protein has a unique sequence, and the human body makes tens of thousands of different proteins. Remembering all of these sequences would be impossible — but luckily, we don’t have to. Nature has already done it for us. The recipes for every protein the body might ever need are stored in our DNA, a vast genetic archive tucked into the nucleus of each cell.
Think of DNA as a long string of bases: four distinct molecules with the names Adenine (A), Thymine (T), Cytosine (C) and Guanine (G). In human cells, the DNA string is about 3 billion bases long in total. This corresponds to around 45,000 unique genes, which are the unique sequences of bases responsible for the creation of your skin, bones, blood, tissue, and everything else in your body, including proteins.
When a protein is needed somewhere in the body, our cells copy the sequence of that particular protein from the DNA and transcribe it onto a short-lived molecule called messenger RNA (mRNA). Just like DNA, RNA is also made up of four different bases (A, U, C, G —> the only difference is that the ‘T’ of DNA is replaced by a ‘U’ in RNA) that represent genetic information. In that sense, it is like a cousin of DNA that has a much more fleeting presence in the body.
I like to compare the process — called transcription — to a book and a photocopy. In this analogy, the DNA is like a book: permanent and large. In contrast, the RNA is like a photocopy of a specific page from that book: a shorter, ephemeral snippet of the larger whole.
Once the ‘photocopy’ of a particular protein has been made, that RNA strand then travels outside the nucleus, where structures in the cell called ribosomes read the recipe and assemble the protein, one amino acid at a time.
This flow of information: DNA → RNA → Protein, is known as the central ‘dogma’ of molecular biology. It is the basic logic of life: code becomes message, message becomes molecule.
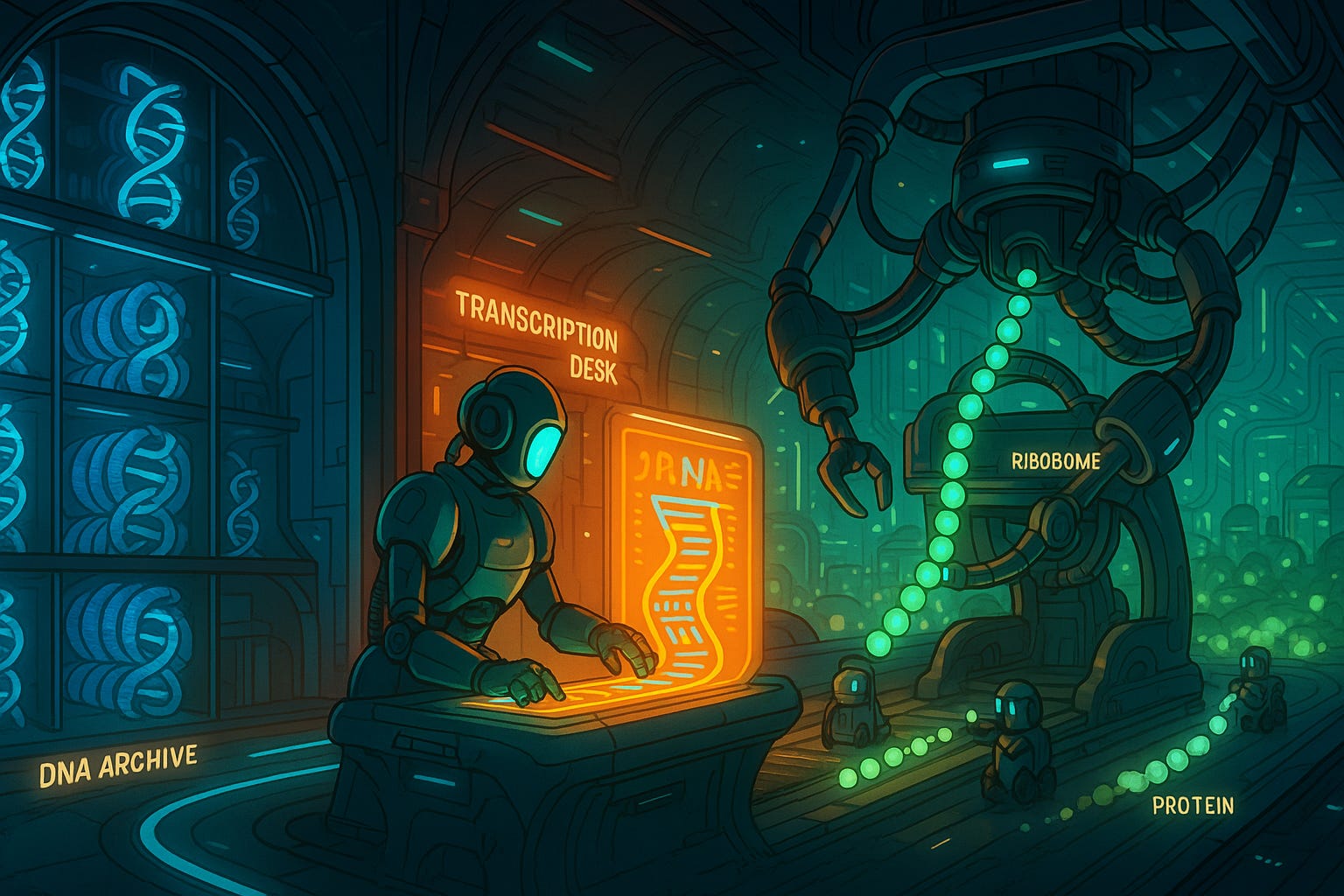
Thanks to this elegant pattern, our cells are able to produce the full toolkit of biology, on demand, every second of every day. Here are just a few examples of different proteins and how they affect the functioning of our bodies:
Myosin & Actin: These proteins are responsible for muscle contraction.
Fibrin: A protein that creates clots to stop bleeding. Absence or defect in this protein results in hemophilia (when wounds don’t stop bleeding).
Hemoglobin: A more familiar name. Responsible for carrying oxygen around blood.
Lactase: If you don’t have enough of this protein, you can’t break down the lactose found in milk, cheese, or yoghurt. AKA lactose intolerance.
Dystrophin: Defects in the dystrophin protein lead to muscular dystrophy.
TNF-α: If you have too much of this protein, you suffer inflammation and run the risk of developing rheumatoid arthritis, IBS, or psoriasis.
In viruses, the mechanism of harm is also a protein. Typically, this is a spike protein on the virus’s surface that jams into cells and initiates infection. That spike is the antigen: the foreign particle that the immune system learns to recognize the shape of and fight off. The actual foot soldiers in this fight are cellular creations called antibodies — which are also proteins, of course — that bind to the antigen and block it from interfering with cells.
This is how your immune system works: it recognizes the shape of foreign objects and prepares an army of defenders designed to counteract those shapes. And vaccines help the body to pre-emptively raise this army by showing it those shapes ahead of time.
So the ultimate goal of PopVax's vaccine programs is to design such a shape — an antigen that closely resembles a real viral spike protein.
🚚 How do we deliver therapeutic proteins into the body?
Designing the right protein is only the beginning. You still have to create that protein and deliver it to the relevant location within the body. This is easier said than done, and the challenge is compounded by the fact that our current protein manufacturing technology is severely limited.
In theory, we can chemically synthesize proteins by stringing amino acids together in the right order. In practice, that only works for small chains called peptides which are typically no longer than 50 amino acids. Since most functional proteins are much larger, with sequence lengths in the hundreds or even thousands, chemical synthesis leads us down a dead end.
Fortunately, there does exist a perfect machine that is optimized for protein production - the cell.
Billions of years of evolution have left cells capable of efficiently cobbling together all manner of proteins; even titin, a muscle protein with 34,000 amino acids in its sequence! So instead of building proteins in a lab or factory, we often engineer living cells to produce them for us. Thereafter, we have three main techniques to deliver them into the body.
In the first method, we farm these proteins externally and then inject them into the body. This approach is known as recombinant DNA technology. It works by using special enzymes to modify the DNA of bacterial cells or mammalian cells so that they include a gene which encodes our desired amino acid sequence. These modified cells are then grown in commercial bioreactors before being harvested for the expressed protein.
The company that pioneered this technology was Genentech in the 1970s. They genetically modified the bacterium known as E.coli (of food poisoning fame) so that it included a gene for one of most valuable proteins of that era - insulin. Before Genentech figured out how to get bacteria to synthetically manufacture insulin for us, the only way to get this critical enzyme was to extract it from the pancreases of dead animals. Not that this was a big deal or anything, the input requirement for one adult diabetic’s annual dose of insulin was just a measly… 30,000 pig pancreases!

A second method to get therapeutic proteins into the body is to make them inside the patient’s own cells using DNA. In this method, DNA that encodes for a certain protein is inserted into the patient’s cells using viral vectors — genetically modified viruses that have their harmful viral DNA replaced by therapeutic genes of our choosing. Leveraging the natural properties of viruses to infiltrate cell nuclei, a viral vector is kind of like a reverse-Trojan Horse that repurposes an ‘evil’ delivery mechanism to deliver ‘good’ custom DNA. Once inside, the cell’s own machinery reads the DNA, transcribes it into RNA, and translates it into molecules of the target protein.
This is how many vaccines work, including the Covishield vaccine that was widely employed in India. In the case of Covishield, scientists reprogrammed a chimpanzee adenovirus to deliver a gene encoding the Covid spike protein into human cells. They chose this virus for three reasons: i) adenovirus only causes a mild cold after infection, ii) humans are similar enough to our primate cousins that the chimp virus can still infect our cells and iii) if they had chosen a common human virus like influenza, many people would already be immune so the virus would be neutralized before it could deliver the spike protein. In contrast to a chimpanzee virus, which presumably nobody except Jane Goodall has immunity to.
At the risk of digressing slightly from delivery mechanisms for proteins, I just want to expand on another kind of treatment that we deliver using viral vectors: gene therapies. In this class of drug, the short fragment of DNA that is injected by the virus encodes some gene that may be absent or deficient within a patient. One example is Spark Therapeutic’s Luxturna: a drug which is surgically injected into the retinal cells of patients who suffer from inherited blindness due to a mutation of the RPE65 gene. Due to this mutation, these patients cannot correctly produce the protein that is responsible for sensing light. However, after receiving Luxturna, these patients’ cells have a copy of the correct RPE65 gene, so their cells make the right protein and the patients gain the ability to see!
Importantly, this is not the same as CRISPR. Gene-editing techniques like CRISPR work by altering or rewriting a patient’s DNA in the chromosomes: the long molecules that are billions of bases long that contain all our genes. Gene therapies like Luxturna don’t do this. Instead, they add a new, much shorter piece of DNA (namely a correct copy of the RPE65 gene) that usually stays separate from the chromosomes. Unlike the chromosomes, this new DNA doesn’t always get copied when cells divide, so its effects may fade over time. This is why some gene therapies need to be topped up every few years. Isn’t biology intoxicating?! OK, digression over.
The third approach to delivering proteins is what PopVax uses: mRNA. Rather than injecting foreign genes into cells or growing proteins in vats of bacteria, they dose the patient with an injection of RNA molecules. This leverages the second step of the central dogma of biology: RNA gets turned into protein.
So when the RNA arrives in the cell, the ribosome reads its sequence and produces the encoded protein, discarding the RNA after the job is done. This is the difference between RNA and DNA therapies: the RNA is a short-term instruction to make a protein that lasts only until the RNA degrades.
In contrast, the DNA that gets injected by viral vectors can stay in the cell for weeks (such as in the case of Covishield) or years (in the case of something like Luxturna). Of course gene editing permanently alters the chromosome so the effects are lifelong. While this sort of long term alteration can make sense when a patient needs an ongoing supply of a given protein, such as RPE65 for sensing light, it doesn’t make sense when the patient only needs a quick dose of a therapeutic protein. And for a vaccine to work, the body just needs to be exposed to the viral spike protein briefly.
So this is the core of PopVax’s strategy: design a synthetic protein that looks like a real viral threat; encode it into mRNA; deliver it into the body; let the body produce the protein; and let the immune system learn its shape.
Everything that follows is in service of making that sequence of events possible.
With that understood, we can begin to understand Popvax’s process and enabling factors in earnest.
👩🏼🎨 Step 1. Designing The Antigen
As an example, let’s imagine that PopVax is seeking to create a flu vaccine. The company’s first task is to come up with a protein that closely resembles the spike protein of the influenza virus.
However, this alone isn’t enough. There are many strains of the flu; viruses mutate, after all. Their spike proteins sometimes shift enough to slip past our existing immune defences — explaining how you can get the flu multiple times, even within the same year. Or how the Delta-variant of Covid ripped apart a country that had already been largely inoculated against an earlier variant.
This is why PopVax doesn’t aim to replicate one exact spike. Instead, they design multivalent antigens: synthetic proteins that combine the essential features of many viral variants. By training the immune system on these broader shapes, the vaccine becomes more resilient to viral mutation and more protective across different strains of the flu.
📐 So How Do You Design Such Shapes?
In short, with computers and machine learning.
Structure prediction tools like AlphaFold, ESMFold, and Rosetta have radically changed the game. These models can take a raw amino acid sequence and predict what structure it will fold into, often with near-perfect accuracy. We use the term ‘fold’ here because it describes how this string of amino acids actually bundles up into an unique 3D shape after construction, the same way an empty crumpled chocolate wrapper will scrunch up after you stretch it out and then release the tension.
In addition to structure prediction tools that can predict how a given sequence of amino acids will fold once they are made into a protein, other tools like ProteinMPNN and Chroma, can do the reverse: given a desired 3D structure, they can suggest amino acid sequences that are likely to fold into that shape.
PopVax, which also employs their own proprietary models, uses both these approaches. They sometimes start from a known spike protein and modify it; other times they generate entirely new shapes based on small structural motifs shared across many viral variants.
This process, although based on computational tools, is not a binary black-and-white exercise; it’s an iterative balancing act to get a protein structure that has several desirable features and minimal undesirable tradeoffs. Eventually, the team lands on a protein shape they believe will do the job — a folded chain of amino acids with just the right configuration to trigger a broadly-protective immune response.
🔁 From Shape to Sequence
Once the protein structure is finalized, the next step is to encode it.
As we know, proteins are made of amino acids. Each of these amino acids is represented in DNA as a unique arrangement of bases, which are the four different DNA molecule types: Adenine (A), Thymine (T), Cytosine (C), and Guanine (G). To be more specific, each amino acid corresponds to a three-letter string of bases called a codon. There are 20 standard amino acids, and each one can be encoded by multiple codons. For example, the amino acid glycine can be represented by GGT, GGC, GGA, or GGG.
By translating the amino acid sequence into a string of codons, and optimizing it for expression in human cells, PopVax generates a DNA sequence that can be used as the template for making the protein.
Here’s what that looks like:
ATG GAA TTC GAC GGT AGA GGG AGA TCG ATG GGA CCT GAC GGT AGA CTC GAG TCG ... (these sequence lengths can reach tens of thousands of characters)This is the genetic instruction set for the synthetic antigen they’ve just designed.
And the entire process — from structural modelling to codon optimization — happens entirely in software.
🩼 Supporting Technologies Enabling Protein Design
This foundational step in Popvax’s process — designing a synthetic antigen in silico (on a computer) — would have been unthinkable just a decade ago. Not because the biology was unknown, but because the tools simply didn’t exist.
For years, we knew how to encode amino acid sequences in DNA and express them in cells. What we couldn’t do was predict how those sequences would fold into actual physical proteins. And since the three-dimensional structure of a protein determines its function, designing proteins without that knowledge was like painstakingly writing an algorithm which would always add some randomness to the output.
But for decades, that’s precisely what we had to do. Resolving protein structures with high accuracy and resolution required gruelling work like x-ray crystallography — a laborious process that took months or years, and often failed.

Consider that by 2020, at which point over one hundred years had passed since father and son duo of William and… William Bragg won the Nobel Prize in Physics for their work in crystallography, humanity had only experimentally determined the structures of around 170,000 proteins. In contrast, the number of proteins that exist in nature is estimated to be in the hundreds of millions if not billions.
So we had but covered a drop in the ocean of protein structures.But in November 2020, there was a sea change.
It came in the form of DeepMind’s AlpaFold2.
It’s a game changer. This will change medicine. This will change research. This will change bioengineering. It will change everything. - The Nature news report about AlphaFold2.

Finally, we had a machine learning model that could predict the structure of many proteins from sequence alone, with near-laboratory accuracy, in just minutes. It was a watershed moment; not just because of its incredible performance, but because it showed that biology could be transformed by the same kind of neural network architectures that had revolutionized other fields.
Suddenly, some of the best minds in AI and protein science converged. The exciting thing is that the fruits of this convergence will only begin to compound and accelerate now.
A couple of years after the release of AlphaFold2, the reverse kind of model followed: ProteinMPNN, a model that could go in the other direction — from shape to sequence.
Taken together, these tools turned protein design from a slow, artisanal handicraft into a generative process in which you can sketch out some desired structural constraints and let the model propose viable biological solutions.
Startups like PopVax can now explore thousands of candidate designs in silico, pick a few that score well, and move directly to testing, with high confidence that the structure predicted in software will hold up in the lab.
This is why Sir Demis Hassabis, co-founder of DeepMind and newly minted Nobel Laureate, has suggested that we may solve disease in the not-too-distant future: because proteins — the core machinery of life — are now legible and engineerable. But why take it from me, hear it from the man himself.
The impact on biology from these advances is profound — but the impact on who can do biology may be even greater.
What used to take a postdoc, a wet lab, and a three-year grant now takes one skilled person and a laptop. Vaccines, chemical enzymes, cancer therapies — all of them can now start as digital artifacts.
This is what we mean by digital biology: when the first meaningful step of a drug isn’t culturing a cell, but writing code.
But design is only half the journey. The body doesn’t respond to bits. It needs atoms and molecules.
⚗️ Step 2: From Code To Cell
At the end of Step 1, PopVax has designed a synthetic protein and generated the DNA sequence that encodes it. But if the goal is to get this protein into the body via mRNA, why are we even making DNA in the first place?
Well, it’s because RNA is extremely brittle and difficult to handle; it is much easier to just make DNA and then rely on the central dogma of molecular biology: DNA makes RNA, and RNA makes protein.
So the idea is to first make physical strands of DNA, called a template, and then leverage natural processes to turn that DNA template into the RNA that the cells will read.
But at this point, the template doesn’t yet exist in any real form. It’s still just text — a sequence of A’s, T’s, G’s, and C’s stored on a computer. PopVax now needs to turn that code into actual genetic matter.
🛍️ Shopping At The Gene Store
Thanks to the marvels of modern science and supply chains, this step has become as simple as any API call or e-commerce order, no more complicated than ordering a customized pizza.
To get their goods, PopVax sends the sequence to a San Francisco-based company called Twist Biosciences, which uses advanced nanotechnology to chemically synthesize DNA, one base at a time. For around $300–400, Twist gets the desired gene printed, packaged, and shipped to Hyderabad in a couple of weeks.
What arrives in the mail is a small, circular loop of DNA called a plasmid. Think of it like a seed which PopVax can plant, grow, and then harvest. The exact way in which this is done will be made clear momentarily, but for the time being just know that the plasmid contains:
The gene encoding the antigen. This is the DNA snippet that corresponds to the viral spike protein that PopVax designed
A selectable marker, which is another gene that we’ll explain shortly
Restriction sites — these are analogous to those little ‘cut here’ markings on plastic food packaging
But Popvax only receives a few micrograms of this plasmid — just enough to get started. To run experiments or prepare for production, they need to amplify it: to make many, many more copies.
And for that, they turn to good ol’ E. coli.
🦠 Cloning the DNA in Bacteria
To scale up the supply of DNA, PopVax introduces the plasmid into bacteria using a process called heat shock. This involves briefly raising the temperature of the bacteria, adding the plasmid solution, and then rapidly cooling the bacteria down again.
The heating step has the effect of making the bacterial cell membrane temporarily porous, allowing some plasmid molecules to slip inside. When the bacteria cool down, the membrane stabilizes once more — ideally with a plasmid safely inside.
But there’s no guarantee that every bacterium will successfully absorb a plasmid — it’s a hit-and-hope process, a bit like tossing a handful of coins at a row of arcade machines and hoping some land cleanly in the slots. Fortunately, the plasmids that do land into bacterial cells can be easily identified and separated from the rest.
The credit for this goes to the selectable marker built into the plasmid. In Popvax’s case, that marker is a gene that confers resistance to an antibiotic called ampicillin. Since the antibiotic gene is built into the plasmid, all bacteria that have taken up the plasmid will display antibiotic resistance; the others will not survive ampicillin exposure.
Leveraging this dynamic, PopVax places the bacteria onto a nutrient-rich plate infused with ampicillin, on which only the plasmid-positive cells will survive and grow.
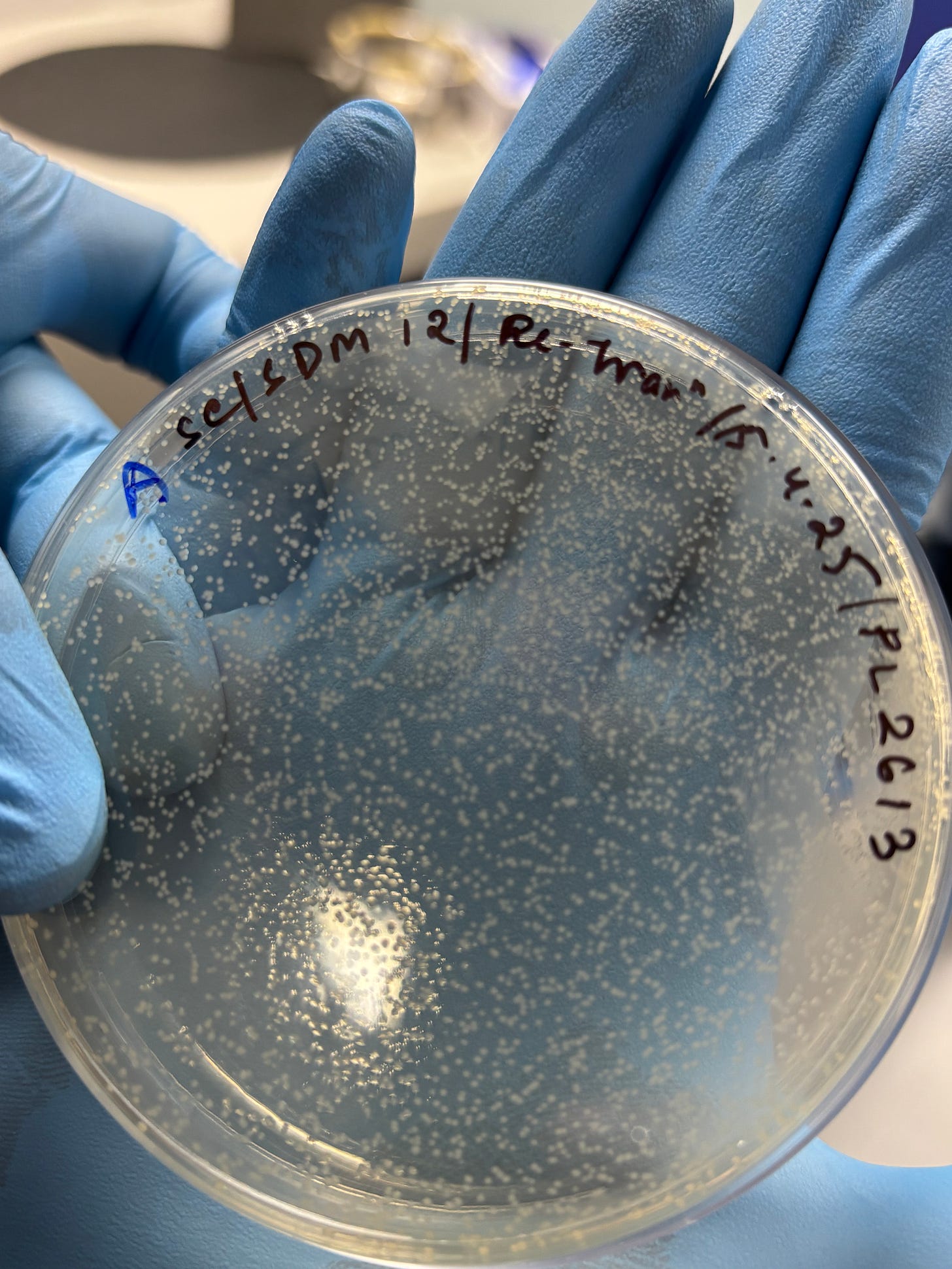
Finally, PopVax is left with a clean population of transformed bacteria, each carrying the plasmid. As these bacterial cells divide, they create copies of the plasmid, giving PopVax a reliable biological factory to scale up production of their custom genetic material.
Overnight, a single bacterial colony can grow into a dense culture containing billions of cells — and milligram-scale quantities of the desired plasmid DNA.
🧪 Harvesting the Plasmid
Once the bacteria have grown to a sufficient volume, the DNA can be harvested.
To do this, Popvax treats the culture with a series of enzymes that break open the cells and extract their contents without damaging the precious DNA inside.
Now PopVax has something like a bacterial soup - a mixture that contains bacterial material, enzymes, the plasmid, and other cellular debris.
The scientists’ task now is to extract their specific gene from the circular loop of DNA that is the plasmid. They are able to do this using clever genetic engineering and naturally occurring enzymes.
Do you remember how we said that DNA is just a long string of A’s T’s, C’s, and G’s?
Well, it turns out that nature provides us with the tools to read, write, and manipulate these genetic letters. To be more specific, there are numerous enzymes that cleave DNA at specific points depending on their genetic code: these are called restriction enzymes and they are foundational tools in genetic engineering.
Let’s make this come alive with an example. Consider the restriction enzyme called EcoRI. This enzyme cuts a piece of DNA whenever it encounters this sequence of bases: GAA TTC. That sequence is known as the enzyme’s restriction site.
Similarly, the enzyme known as XhoI has the following restriction site: CTC GAG.
By leveraging the known properties of these restriction enzymes, scientists can build plasmids that are easy to harvest DNA from. So when Twist Biosciences builds a plasmid for Popvax, they sandwich the custom gene that Popvax ordered between two restriction sites.
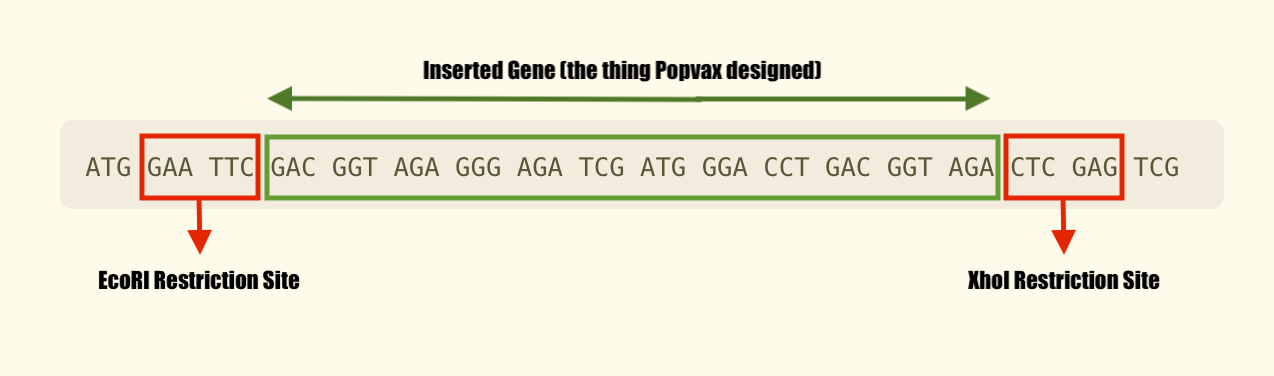
All Popvax has to do to chop out its desired gene is introduce the two restriction enzymes into the bacterial soup mixture, and let them automatically go and cleave the plasmid DNA at their respective chopping blocks.
The result is the emergence of the exact DNA strand PopVax designed in software, except now tangible and real. But it’s still floating in a complex mixture alongside everything else that was inside the bacteria!
To separate it, PopVax places the solution in a silica column — an empty plastic test tube fitted with a kind of sieve made from silicon — and loads it onto a centrifuge.
The column is treated with a specific mixture of materials intended to stick only to the DNA, so when the centrifuge spins, everything but the DNA is pushed to the bottom of the tube. Sort of like applying some pressure to a sieve that forces small particles and liquids out the bottom while leaving larger materials on top.
PopVax then extracts the DNA from the silica column and gives it one final wash with ethanol to make sure that it is free from any contaminants.
What they end up with are some high-purity strands of physical DNA that are ready for the next step in the chain: transcription into mRNA.
💡 Why Not Just Order The DNA Strands Directly?
While it is technically possible to order bulk quantities of purified DNA — or even RNA — from synthesis providers, PopVax chooses to handle this process in-house. The reasons come down to cost, control, and flexibility.
Cloning plasmids internally is far cheaper than outsourcing, and it allows the team to rapidly tweak designs, scale production as needed, and run their own quality control. It also avoids the logistical complexity and cost of cold-chain shipping, especially for RNA, which is fragile, expensive, and degrades quickly in transit.
Just as importantly, owning this step helps Popvax build internal know-how and reproducibility — critical factors as they move from R&D to clinical-grade manufacturing. In modern biotech, the ability to go from design to molecule in a single room isn’t just efficient, it’s a strategic advantage.
🩼 Supporting Technologies Enabling DNA Synthesis
The best way to understand what makes this step possible for a small company like PopVax is to look at the Carlson Curves, named after bioengineer Rob Carlson. These are the biotech equivalent of Moore’s Law: they track the cost of sequencing and synthesizing DNA over time.
And like Moore’s Law, the Carlson Curves demonstrate an exponential drop in the price to sequence and synthesize a DNA base over time.

To put things in perspective, consider the Human Genome Project — the most ambitious biological collaboration in history. It took 13 years, 20 institutions from six countries, and nearly $3 billion to sequence the human genome for the first time. When the project was declared “complete” in 2003, 92% of our genetic code had been mapped, base by painstaking base.
Today, a £5,000 desktop device from Oxford Nanopore can continually sequence entire genomes in under 30 minutes each. As the technology keeps improving, it’s hard to imagine a future where genome scans aren’t as routine as blood tests.
Some futurists have even floated the idea of “smart toilets” — bathroom fixtures that quietly analyze the genetic material in your waste to monitor your genetic health, flag infections, and keep tabs on the ever-shifting ecosystem of bacteria in your gut.
Just like the cost of sequencing DNA has fallen, the cost of making DNA has followed a similar arc too. Companies like Twist can now print thousands of custom DNA strands — each thousands of bases long — for less than $200 a piece. We’re still far from being able to write an entire human genome from scratch, but tools like CRISPR provide us with incredible capabilities in the interim2.
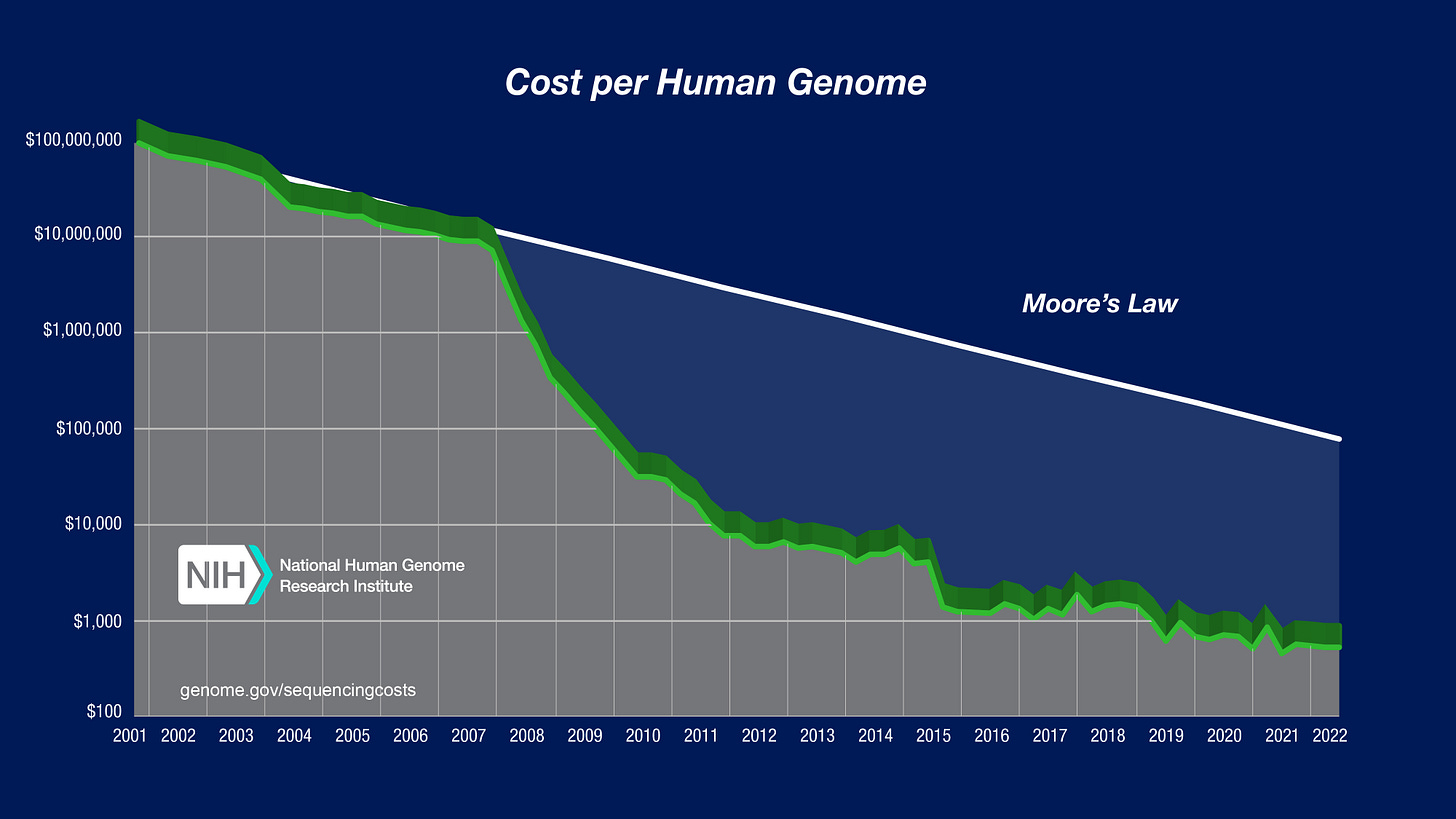
The result of these cost collapses is that the highly specialized nature of working with DNA has now become routine. Gene synthesis is no longer a bespoke laboratory procedure, it’s a cloud service.
A startup PopVax can go from a digital sequence to a shipped, lab-ready plasmid in a week or two, which allows them to move fast and test new ideas quickly, cheaply, and continuously.
It helps that everything else — from restriction enzymes to centrifuges — has also become cheaper, modular, and more reliable. Taken together, these advances make it very easy for newcomers to get started with genetic engineering.
So once Popvax has their custom strand of DNA, they proceed to the next step → turning their DNA strand into RNA.
🧬 Step 3: From DNA To RNA
Now that PopVax has a clean, purified strand of DNA, the next step is to use it as a template for making mRNA — the molecule that will ultimately travel into the patient’s cells and initiate the creation of PopVax’s antigen.
In biology, this process is called transcription, and it’s how all cells turn long-term protein recipes stored in DNA into temporary, functional instructions to order a particular protein. Inside our cells, transcription is done by an enzyme called RNA polymerase, which binds to DNA, reads the sequence one base at a time, and assembles a matching strand of RNA.
In PopVax’s case, this happens in vitro, meaning in a test tube. Hence the term: in vitro transcription, or IVT.
🧫 Copying the DNA into RNA
To run IVT, PopVax mixes the following key ingredients in their test tube:
The DNA strand containing their gene of interest that they just finished isolating and purifying
An RNA polymerase harvested from a virus that infects bacteria
A supply of RNA bases for the polymerase enzyme to use as building blocks. These are just like the molecules that make up DNA: A, T, C, and G. The only difference is that RNA has a base called Uracil (U) instead of the Thymine (T) found in DNA.
A few supporting chemicals to help stabilize the overall mixture and allow the reaction to go smoothly
Once these ingredients are combined, the RNA polymerase takes centre stage. It binds to the DNA template and begins to ‘read’ its sequence. As it does so, it grabs the RNA bases that are floating nearby and merges them into a single strand that is an exact copy of the DNA template, except in RNA form.

But transcribing the RNA is only part of the job. Turning it into something usable — and safe — requires more work.
🧼 Cleaning Up The Mixture
The first step towards this is to isolate the freshly created RNA strands from all the other stuff in the test tube.
In parallel fashion to the DNA harvesting step, a silica column packed with materials intended to stick to RNA can be used here to separate the RNA from the enzymes, bases, and other items floating alongside it.
But there is a catch! There is another item in the test tube with similar properties to the RNA: the DNA template it was copied from. This makes separation difficult, since the DNA might also stick to the silica column.
To get around this, PopVax leverages yet another useful enzyme provided by Mother Nature: DNase (pronounced like D-N-Ayse). This enzyme breaks down DNA strands while leaving RNA intact. Popvax uses this enzyme to degrade the DNA template in their test tube, which allows them to confidently put the mixture through the column and harvest the output: high-purity RNA.
But before this molecule can be delivered into organisms, there is one final step: the lipid nanoparticle.
🩼 Supporting Technologies Enabling RNA Preparation
The science behind in vitro transcription has been around since the 1980s, but for a long time, its use in human medicine was considered unworkable. Synthetic mRNA triggered strong immune responses, degraded rapidly in the body, and often failed to produce meaningful protein levels inside cells.
That changed in 2005, when Katalin Karikó and Drew Weissman (who would go on to win the Nobel Prize in Physiology in 2023) discovered that swapping one of RNA’s natural building blocks — uridine, basically a form of the base uracil (U) — with a chemical analogue called pseudouridine dramatically reduced immune activation while improving stability.
This single modification made mRNA therapeutics viable for the first time, and was the key breakthrough that enabled the success of COVID-19 vaccines.
Thanks to that discovery — and to the expanding ecosystem of enzymes, kits, and purification tools that followed — PopVax can now run IVT reliably, reproducibly, and with confidence that what they develop in a test tube has a high probability of safely producing the right protein in humans.
A process that once took months in specialized labs can now be done in hours, on a benchtop, by a small team. But even with pseudouridine, mRNA cannot be delivered on its own. It still needs to be safely posted to the cell.

📩 Step 4: Making The Envelope
mRNA is precise, potent, and beautifully designed, but without protection, it breaks down in the harsh conditions of the body.
To solve this, PopVax encloses its mRNA in a microscopic protective shell called a lipid nanoparticle, or LNP.
LNPs are tiny, soft-shelled spheres made from fat-like molecules that serve two essential functions: they shield the fragile mRNA, and they help it cross into cells, where it can be translated into protein. Without them, the entire platform falls flat.
The LNP is made by bringing together two components: the mRNA and a blend of lipids. PopVax does this using a technique called crossflow micromixing, in which the two mixtures are pushed through carefully engineered, pressurized channels that cause the two liquids to mix effectively.
Within these precisely controlled, high-velocity streams, the lipids and mRNA spontaneously self-assemble into uniform particles, encapsulating the RNA in a protective lipid envelope in just a matter of milliseconds.
Once formulated, the mRNA-LNP mixture is run through a final sterile filtration step, removing any impurities or particulates. The resulting liquid is the finished product: a stable, injectable solution, containing billions of RNA-filled particles, each one capable of slipping into a human cell and delivering a carefully written message.
At this stage, PopVax’s digitally designed antigen has been translated into a physical RNA sequence and packaged in a robust delivery vehicle. It is now ready to enter the body and start teaching the immune system what to remember.
🩼 Supporting Technologies Enabling LNPs
Many medical treatments have long been held back by the issue of delivery; it is fundamentally difficult to get large molecules like DNA strands inside of cells.
This changed in 2008 thanks to a major breakthrough by a lab from the University of British Columbia. Led by Dr. Pieter Cullis, this Vancouver-based group developed ionizable lipids, special molecules that could bind to mRNA and then release it inside cells.
It is precisely this invention that allowed scientists to begin actually delivering RNA molecules into cells. The LNPs used in Moderna and Pfizer’s COVID-19 shots were direct descendants of this work.
Alongside this new chemistry came the tools to make it work at scale:
Microfluidic mixers that enabled high-efficiency, reproducible encapsulation
Particle size analyzers, to ensure the uniformity of the nanoparticles
Today, these systems are commercially available, but it is still rare to find safe, high-efficacy ionizable lipids that can be used in LNP formulations. When making their Covid vaccine, Pfizer & BioNTech had to license their ionizable lipid from Acuitas, one of Cullis’ companies.
The other major mRNA vaccine manufacturer, Moderna, had to painstakingly develop their own ionizable lipid.
Just like Moderna, PopVax has brought the ionizable lipid platform in-house. Their work in this arena is led by Dr. Maunish Barvalia, who trained under Pieter Cullis himself. This effort seems to have yielded great dividends; after an exhaustive discovery process in which they developed their own library of 600+ ionizable lipid designs, PopVax found a unique formulation that equals Moderna’s market-leading product when it comes to safety and delivery performance.
So thanks to the work of these scientists and the LNPs they create, PopVax’s RNA gets safely enveloped and prepped for delivery into cells. Which brings us to the final part of the process, actually testing the vaccine.
🐁 Step 5: Test Is Best
Once PopVax has their mRNA molecule safely wrapped up in a lipid nanoparticle, the final step is to see if the synthetic antigen actually grants immunity.
This is done by injecting the vaccine into mice, and tracking the immune response over time: which antibodies are produced and in what quantity, and what are those antibodies able to do to the virus the vaccine is designed to combat.
This isn't just a safety check, it's a feedback loop. By comparing the antigen they designed to the antibody response it generates, PopVax gathers invaluable data. They are then able to use that data needed to refine their models.
But once a candidate shows strong immunogenicity — that is, it provokes the right kind of immune response — PopVax tests it for toxicity. They inject a full adult dose into rats, then observe them over time for adverse effects. The end result is a data package that captures the vaccine’s performance in mice and rats.
Still not quite the same as human trials, but about as close as you can get in early-stage science.
V. ⏩️ What’s Next for PopVax?
Now that they have their shot, where does the company go from here?
Now that PopVax has two ready jabs with promising data — namely their broadly-protective influenza and COVID-19 vaccines — the logical next step would be to get these treatments into humans.
The ideal route would run through the U.S.A; it is home to the FDA3, still the world’s most respected regulatory body, and is also the market with the highest drug prices. If you get a product approved in America, you’ve already won the most important battle.
This is why PopVax is gearing up for Phase I trials of their COVID-19 vaccine in early 2026 in the USA. This trial will be conducted and sponsored by the National Institute of Health (NIH), which means that the agency will cover the few million dollars of costs and manage the actual patients. All PopVax has to do is hand over the vaccines, and wait to receive a package of data in return.
Together with the company’s $2 million prize from the US BARDA to develop their flu jab into an arm-patch delivered vaccine, this signals excellent prospects for PopVax in the American market.
But things are slightly more complicated than that.
America is currently…. going through a moment. Vaccine skepticism is on the rise; 28% of the population believes that Covid vaccines caused a wave of deaths that simply never happened. Only 69% of respondents in this Gallup poll believe it is very important to administer vaccines to children, down from 94% in 2001.
Hell, some parents are even organizing ‘measles parties’ for their kids to get exposed to the virus, even though the data shows that measles can cause hospitalization, brain swelling, and death in a significant proportion of children.

Given this growing public skepticism of vaccines, investor interest in this category has cooled slightly. There have been better years than 2025 to be selling vaccines in America.
For PopVax, these developments reiterate the need for diversification.
While the US market and regulators are still Popvax’s priority, the company is also planning to begin human trials for their flu shot in India early next year.
Moreover, they are working hard to improve their technological capabilities. Specifically, they’re doubling down on the thing that sets them apart from most other companies in their field.
🖥️ Building The Immunological Compiler
PopVax was never just a run-of-the-mill biotech; its founder is a computer scientist, after all. Its core competence is in computational design and machine learning, skills that enable it to take aim at a problem that sits at the heart of immunology.
Recall that today we design antigens — fragments of viruses or foreign particles — and inject them into the body, hoping that they trigger the production of antibodies. It’s the antibodies that actually do the fighting on behalf of our immune system; they bind to antigens, block them from entering cells, and mark them for destruction.
But here’s the thing: we don’t design the antibodies themselves. The body does. We cannot reliably predict what exact antibody will be produced in response to a given antigen. All we know is that there are billions of possible variants of antibodies, and that the immune system mass-produces some of these variants when it sees an antigen. But the exact mechanism behind this remains something of a black box.
It’s not dissimilar to a programmer writing Python and hoping that the compiler turns his high-level code into good machine code, without knowing how the compilation process actually works. And while that kind of approach can still result in productive software, it is a bit of a blind gambit. In comparison, deep knowledge of the inner workings of the compiler allows engineers to write code that is much more efficient, flexible, and potent.

This is why PopVax is trying to build this immunological compiler: a predictive engine that tells us which antigens will lead to which antibodies. To do this, they are injecting synthetic antigens into mice and analyzing what kinds of antibodies they produce. The hope is that with a large enough dataset, machine learning will reveal the mysteries of the immune system.
If that works, it would be a game changer.
Because while antibodies already form the basis of some of the most powerful drugs we have, we have never truly designed them. We sort of just borrow them from places and then transplant them into patients.
To understand the meaning behind this, as well as the limitations of this approach, take the example of anti-venom. We produce this life-saving biologic (a molecule/drug that is made by or found in natural organisms) by dosing horses with small quantities of snake venom. Inside the horse’s body, the antigen in the snake venom (a wicked sounding protein called disintegrin) causes the horse’s immune system to produce antibodies. We then harvest these antibodies from the horse’s blood and inject them into snakebite patients, thereby helping to neutralize the antigens causing those patients harm.

In addition to animals, human antibodies form the basis of many treatments too. The monoclonal antibody treatments that you may have heard about during the pandemic worked according to the the same principle. Famously, Regeneron used antibodies from COVID-19 survivors to create injections that helped patients boost their immune defences against the virus. Donald Trump received this treatment when he fell ill.
Although these techniques undoubtedly provide us with many benefits, they also have drawbacks. The therapeutic antibodies have to first be isolated from survivors or grown in bioreactors. From there, they have to be purified, shipped in cold storage, and then repeatedly administered to patients intravenously. It works, but it’s expensive, slow, and painful.
A case in point is Keytruda, the cancer immunotherapy that nets Merck a record-breaking $30 billion a year (half their annual revenue). This drug, a monoclonal antibody treatment that ultimately comes from rodent cells, is priced at around $150K annually in the US (with per-dose manufacturing costs estimated to be ~$300). It also takes 30 minutes of sitting hooked up to an IV to receive one dose.
In contrast, it can cost just a few dollars to manufacture a single mRNA dose. The vaccine can also be delivered with a single jab, sometimes two, and has the additional benefit of using the patient’s own body as the factory for the therapeutic antibodies.
This typically results in longer and more robust immune protection in comparison with antibodies from outside. The reason is that producing antibodies from one’s own cells actually teaches the immune system how to respond to a threat, allowing those antibodies to be rapidly produced from immune memory if the body encounters that antigen again. In contrast, importing antibodies from another donor is a more temporary solution that works well in urgent scenarios but not for long-term protection.
All of these facts point to the massive potential of vaccines to help combat disease.
But that might only be the tip of the iceberg. The immune system doesn’t just fight infection, it also maintains internal order. And sometimes, that order collapses with catastrophic consequences.
Autoimmune diseases like type 1 diabetes, multiple sclerosis, or celiac disease occur when the immune system misidentifies the body’s own tissues as threats and produces antibodies that attack them. Allergies work the same way, but in reverse: the immune system overreacts to harmless substances like peanut proteins. In both cases, the body’s immunological gun is firing, but it’s pointed back at the shooter.
The good news is that this response can be reshaped. We already try to do this with oral immunization programs and other treatments. With peanut allergies, doctors expose patients to tiny amounts of the allergen over time to slowly build tolerance. In autoimmune research, early-stage trials in diseases like multiple sclerosis and celiac are experimenting with tolerizing vaccines — antigens that don’t trigger a violent response but instead retrain the immune system to tolerate certain antigens. These approaches are promising, but they’re slow, blunt, and empirical. We’re guessing, and hoping it works.
Now imagine if we had mastery over the black box of antibody creation. Instead of just treating the symptoms of autoimmune conditions, we could halt them at their source. Instead of shutting down our natural defences with immunosuppressants, we could simply rewire them. Instead of having to always skip that pizza, you could use a synthetic antigen to override your system’s rejection of that pesky protein gluten. Just like a software patch that fixes a bug.
But to write that patch, you need to understand how the code works. In other words, you need a compiler. And building that compiler means training a model — not just with theory, but with a massive volume of biological data.
And that’s where RNA’s unique capabilities shine.
🧩 What is RNA’s Unique Advantage?
To answer this question, think about what it takes to train an immunological compiler. You need to generate thousands of antigen–antibody datapoints, each one involving the design, delivery, and testing of a custom antigen.
This begs the question: how do you manufacture so many antigens quickly, consistently, and at scale?
Using the recombinant approach to protein production — growing antigens in mammalian or bacterial cells — would soon lead you into production bottlenecks. After first figuring out through trial-and-error which kind of animal cell is best suited to producing your new antigen, you would have to then grow those cells in a bioreactor before you could harvest and purify their expressed protein. The cells themselves would take a few weeks to grow to a size that could yield sufficient quantities of the desired protein, and even a minor contaminant in the bioreactor could destroy weeks of work. It’s time-consuming, costly, and fragile.
Viral vectors are even worse. Each new antigen means rebuilding the viral genome and revalidating its behaviour in cells, a process that's protracted and labor-intensive. Because viruses can replicate or integrate into the genome, this kind of work is also tightly regulated to manage biosafety and contagion risks. It’s effective for final therapies, but not ideal for rapid experimentation or iterative design. It's like rebuilding the delivery truck every time you want to ship a different package.
On the other hand, mRNA is extremely fast and modular.
Switching from Antigen A to Antigen B in an mRNA workflow is as simple as ordering a new DNA template from a vendor like Twist Biosciences. The rest of the process is exactly the same as we described in Section IV, down to all the chemicals, staff, and machines needed. No need to change a single thing.
The only step involving live cells — the most fickle and tricky component — is the bacterial cloning of the plasmid, which takes one night in a disposable dish. There are no bioreactors, hazardous viruses, or protein purification steps involved. In fact, you’re not even making the antigen directly, you’re just delivering the code for it to get made inside a cell!
It’s as close to copy-paste as it gets in biology. PopVax places hundreds of orders for different plasmids from Twist every month, and they’re able to just plug those packages into their workflow without fuss or customization. A new plasmid gets turned into a jab for mice in barely a week. No other protein production method would come close.
This is why mRNA is perfect for high-throughput iterative testing. Whether for pandemic response for a mutating virus like Covid, or for generating the large dataset for an immunological compiler.
🌱 Seeding Companies Through Tez Bio
The compiler will certainly aid PopVax’s development of it’s next-generation vaccines -– indeed, that’s why they are building it — but the ability to design antigens and manipulate immune responses opens up far more doors than any one company can walk through alone.
Creating the underlying technology is one kind of challenge; translating that into specific therapies for dozens of different diseases is another entirely. It requires deep, indication-specific expertise in the form of scientists and researchers who know a particular disease inside out.
These kinds of specialists surely exist, but many are unlikely to have advanced capabilities in machine learning or protein design. PopVax wants to leverage this dynamic to enable others to build on its own platform; to give them the infrastructure, tools, and support they need to move fast and build well.
That’s the idea behind Tez Bio: a new program PopVax is starting to incubate and support startups that can use their engine to develop targeted therapies across a range of conditions.
PopVax brings the core platform, the wet lab, the delivery system, and the immunological tooling so that these teams can start closer to the finish line. They can even rapidly manufacture doses safe for clinical trial! “Tez” means fast, and that’s the promise: skip the setup, and get straight to the science.
All things considered, this strategy does seem exciting; especially because the conditions for building cutting edge biotech in India are beginning to look very appealing, and the PopVax team are among the few people in India with the expertise to evaluate and guide truly novel therapeutic development efforts.
But before we explore those changing conditions, a big congratulations for making it past the dense and technical part of the essay! You are now invited to momentarily relax your eyes and mind at The Disco Ribosome™️.
VI. 🇮🇳 Why Is This Bullish For India?
Prior to examining the present tailwinds for biotech in India, it’s worth taking a moment to appreciate the country’s history when it comes to biomedical innovation. Honestly, would it even be a Tigerfeathers piece without this?
🛕 Rare Yet Remarkable Historical Context
While many of our readers may be familiar with Ayurveda, I will venture that very few of you would recognize the name of Sushruta, the ancient medical pioneer often hailed as "the father of surgery." Rooted in the broader philosophy of Ayurveda, Sushruta’s work stands apart for its extraordinary attention to surgical techniques, anatomy, and diagnosis.
Sushruta’s magnum opus, the Sushruta Samhita, written in the ancient city of Kashi, is a treasure trove of surgical wisdom. It contains detailed descriptions of hundreds of procedures, anatomical features, and techniques for preventing and treating infections. Despite being written some three thousand years ago, Sushruta’s ideas spread across generations, influencing scientists during the Islamic Golden Age of Science and through them, European physicians of the Renaissance. Remarkably, some of his techniques, like the 'Indian flap' method for nasal reconstruction, are still used in modern surgical practice.

In addition to erm.. inventing nosejobs, Sushruta was also one of the first to suggest that diseases could spread from animals like rats and mosquitoes. To appreciate the gravity of this bold idea, remember that modern germ theory would only gain credence millennia later. In this sense, Sushruta was more than a medical practitioner; he was a pioneer, shaping the future of medicine in ways that would resonate around the world.
And though few young Indians know about Sushruta and his pivotal contributions to world medicine, even fewer probably know that the very concept of vaccines has its origins in the subcontinent!
Centuries ago, long before high-tech vaccinology, India had its own public health workers called tikadars. These learned men would travel from village to village performing variolation, the deliberate inoculation of a patient with material from smallpox pustules. This was a crude and risky practice by today’s standards, but remarkably effective for its time. Some historians contend that the practice originated in India, from where it was carried westward by travellers and traders, eventually coming to the attention an Englishman, Edward Jenner.
Under Jenner’s skilful hand, the idea behind variolation would be adapted; instead of exposing patients directly to smallpox pustule dust, Jenner’s new method would introduce the patient to cowpox pus instead. Contemporary science now tells us that this procedure worked because the spike protein of cowpox is very similar to that of smallpox.
But Jenner wasn’t to know this; his inspiration to try cowpox material came from a chance realization that milkmaids seldom suffered pockmarked faces. Due to their proximity to the cows that carried cowpox, these milkmaids would generally be immune to its deadlier and more disfiguring cousin, smallpox. This is the origin of the phrase ‘as smooth as a milkmaid’s skin’!
Aside from its fascinating backstory, the invention of this vaccine is crucial because it conquered one of the deadliest plagues in history: smallpox. Millions of lives were claimed by smallpox each year, yet this breakthrough liberated humanity from its grip. It always amazes me that the inspiration for this civilization-propelling invention can be traced back to ancient Indian practices!
Almost a century after the creation of the smallpox vaccine in 1796, came another important chapter in the history of Indian immunology. In the late 1800s, Waldemar Haffkine, a Russian-Jewish microbiologist, made Bombay his laboratory. From a modest facility in Parel, he developed the world’s first bubonic plague vaccine, bravely injecting himself to prove its safety.
By 1902, his institute was supplying millions of doses across India and beyond, cementing India’s role in global biomedical innovation. Today, the Haffkine Institute remains a thriving hub of scientific research, continuing to push the boundaries of biomedical discovery. A part of the institute also serves as a museum, a fitting tribute to its remarkable legacy and enduring role in advancing science.

In more recent modern times, the torch has been carried by the Serum Institute of India. Founded in 1966, Serum has become the world’s largest vaccine producer, churning out billions of doses each year. Their impact extends beyond just the pandemic; their vaccines have helped eradicate polio and fight several other diseases. It was right near Serum’s Pune campus where Soham found the spark to create PopVax.
In light of India’s long-standing role in global health, that now seems like a natural progression.
The point of this historical detour is to emphasize that this is not a new path for India. We have innovation and biotech capability in our DNA, we just need to harness it. And luckily, there are a number of factors that are converging to make this a singularly good time to build biotech in India.
⏳ India’s Advantages, Here & Now
There are five major reasons why this country enjoys a competitive advantage relative to the rest of the field in biotech.
1. A Deep Pool of Scientific Talent
Biology is still manual. Despite the advent of AI and robotics, much of the work done in labs today involves pipetting, culturing, synthesizing, and dissecting. In other words, it requires careful hands and trained minds. India has both.
By sheer population, we graduate more biologists, chemists, and lab technicians than almost any country on Earth. And while automation will eventually chip away at this advantage, that future is still distant. For now, the ability to do biology well and affordably is a strategic asset.
And it’s not just that we have a large quantity of scientists; we also have quality researchers too. The kind of folks that staff global labs, lead Big Pharma research groups, and earn tenured positions in leading Western institutions.
Granted, our highest-caliber researchers often move abroad, but now is an opportune time to entice them back. Especially because the prospects for scientists and foreigners in the US are looking increasingly shaky.
The American political leadership is currently withdrawing a lot of scientific funding from labs and institutions in the name of cost-cutting. They are also cancelling green cards, withholding visa approvals, and stopping all new student visa applications until they figure out how to “vet students’ social media profiles”.
As we speak, hundreds of Indian researchers and students at Harvard are gripped by panic over whether the administration is going to kick them out of the country overnight.
For the first time in decades, we have a window to bring top-tier talent home and give them serious science to work on.
2. Manufacturing & Development Infrastructure
India already knows how to manufacture complex molecules; our pharmaceutical majors churn out more generic drugs than any other country on Earth. When we get the formulation for a molecule, we know how to put in place the people and processes needed to achieve global scale.
But in addition to just manufacturing drugs, we can also help develop them. Although they don’t have the same global dominance as their counterparts in manufacturing, our contract research organizations (CROs) help the biggest names in global pharma to discover and refine new drugs.
The likes of Syngene, Aragen and their peers provide research and discovery services to the biggest names in global pharma such as Pfizer, Merck, and Roche. Many times, drug candidates from these Western giants are first synthesized, dissolved, pipetted, screened, and analyzed by Indian hands.
This network of CROs offers a significant advantage to local biotechs. Proximity, ease of access, and cultural familiarity all contribute to faster development timelines and reduced costs.
It also helps that the cost structure of the industry is changing at a macro level; Twist Bioscience is a case in point. They design, create, and operate expensive DNA printers, which customers can simply pay to use on-demand. CROs are the same: they go ahead and buy all the expensive machines needed to synthesize, purify, and measure various drugs and chemicals, and then rent them out through their service models.
This means that the capex costs to get started are reducing. You no longer need huge warchests to build a biotech, you just need access to the stack. And we have both the people and machines that comprise that stack here.
3. A Computationally Literate Workforce
The biggest shift in modern biotech is that the field is becoming increasingly software-first. Everything from drug discovery to protein design to genomics can be boosted by familiarity with code. That plays straight into India’s strengths.
We have a vast and growing pool of engineers, data scientists, and applied mathematicians, many of whom are now turning their attention to biology. As tools like AlphaFold, ProteinMPNN, and biological language models become central to R&D, countries that can bridge biology and computation will move faster.
We’re one of them.
4. Operating Cost Advantages
Despite all the advances in AI, some parts of biology are still inherently physical. Animal testing is one example.
Lab rats and mice must be bred, housed, screened, and monitored under highly controlled conditions. They must also be virgins, meaning they must be microbiologically clean and free of any unwanted food, medicine, or physical interactions that might affect the data they generate.
After being dosed or treated, these lab animals must be observed in detail. None of this can be rushed; biology takes its own time.
Once the treatment has taken its effect, the animals need to then be euthanized, dissected, and analyzed. This involves a lot of pretty manual and grisly steps such as liquifying a rat’s spleen and checking whether it contains certain antibodies or not.
As you would expect, humanity hasn’t built robotic rat-spleen-excavators yet, so all this work falls on humans. Humans who need to be trained and qualified, and who have to work in completely sterile environments.
Remember, the rats must not be contaminated in any way, so they must be housed within airlocked rooms in sterile enclosures with custom ventilation that pipes in only HEPA-filtered air, purified water, and quality-controlled food. The tools and cages touching these animals must also be wiped down and cleaned, a process that once again can only be done by hand.
The same standards apply to all the work done in the lab with cells - it must be done in highly sterile environments.
This is where India’s cost advantage comes into play. Not only are labor costs dramatically lower, but the infrastructure for maintaining these high standards is also much cheaper to build and run in India compared to places like Basel or Boston.
But this isn’t about cutting corners. It’s about a genuine cost-quality arbitrage. As long as biology still requires this level of physical handling—and we’re not yet at the stage where rats and cells can be fully simulated—India has a distinct advantage.
However, this might not last forever. The FDA is already beginning to phase out the necessity for animal testing in drug development, thanks to advancements in lab-grown organs and computational models. But for now—and probably for the next couple of decades—India can perform this work faster, cheaper, and at scale, without compromising on the quality required for rigorous scientific research.
5. A Large Disease Burden
India’s vast disease burden is an undeniable tragedy; millions suffer from conditions that are preventable, treatable, or simply ignored by global pharma. But this uncomfortable reality also gives us a powerful advantage: proximity. The very fact that these diseases are so close to home means we can study them more deeply, understand them in their social and biological contexts, and move faster to develop interventions that work in the real world.
A large, diverse population with a wide range of illnesses offers an enormous base for clinical research. Trials that might take years to recruit in the West can be enrolled here in weeks. We also see firsthand the full spectrum of disease: from early-stage to drug-resistant, from urban hospital cases to rural complications. This gives Indian researchers and companies a unique edge in developing, testing, and refining treatments that are both globally relevant and locally grounded.
Beyond speed and scale, there’s something more important: insight. When the problem lives next door — in your hospitals, your clinics, your own extended family — you approach it with greater urgency and contextual understanding. That’s something no Western research lab or offshore trial site can replicate. For India, the burden is heavy — but it’s also a reason to lead.
🔬 Indian Builders Worth Watching
To an inquisitive outsider, India’s bio-industrial base is still focused on generics and biosimilars. That’s what the big players — like Biocon, Dr. Reddy’s, and Sun Pharma — have built their empires on.
To be clear, that’s not a bad thing. These companies have done vital work. Just look at Cipla and Yusuf Hamied, who helped break the monopoly on AIDS drugs and lift Africa out from the jaws of their horrible epidemic.
Nevertheless, the many benefits of being the ‘pharmacy of the world’ must not dampen our ambitions to push further. We must create new science and new medicine, too. Luckily, there are already a few trailblazers leading the way.
PopVax aside, here are a few of the companies showing what Indian biotech might look like in the next era:
Immuneel, co-founded by Kiran Mazumdar-Shaw of Biocon with the famous physician-writer Siddhartha Mukherjee, and led by scientists with deep experience, is building a platform for immuno-oncology in India — another shot at bringing globally relevant therapies home.
ImmunoACT, spun out of IIT Bombay, is working on CAR-T cell therapies for cancer. Their treatment involves collecting the patient’s white blood cells — specifically their T cells — and genetically engineering them to express a protein that turns them into cancer-hunting cells. Backed by the CRO Laurus Labs, this company provides its cancer treatments for a tenth of the price of US firms.
Eyestem is aiming to restore vision in patients with degenerative eye diseases. They harness stems cells4 to create young, health retina cells that they implant into patients’s eyes. With under $10M raised, they have developed a treatment that is competitive with large Western labs when it comes to restoring vision in patients.
Bindwell is applying protein design tools to create novel bio-pesticides. It’s a reminder that biology isn’t just for humans; we can also program proteins to treat crops, livestock, and ecosystems. This exciting startup helmed by an 18-year-old Caltech dropout from New Delhi even caught the attention of Paul Graham.
These aren’t the only ones. I’m sure there are others, it’s just that my own knowledge and network in this industry is still growing. But I would love to learn more; if you’re reading this and know someone who is building something interesting, do reach out.
VII. 🚧 Risks, Drawbacks, Barriers
It’s tempting, when writing about Indian biotech, to get carried away by the possibilities enabled by the return of talent, rise of new startups, and structural advantages we hold. But it’s important to be honest: this is not an easy field, and the road ahead is full of real risks. If we want to build something lasting, we have to be clear-eyed about the barriers — not just as observers, but as a community that intends to help solve them.
Let’s start with PopVax. The company is promising, the vision is exciting, and the platform is coming together well. But right now, they only have mouse and rat data. Until they get results in humans, not just preclinical models, we have to treat all of it as an advanced hypothesis. That doesn’t mean it’s not worth betting on, but let’s be clear: no amount of beautiful theory and animal data will matter if the drugs aren’t safe or don’t work in humans.
Then there’s the ambition of building an immunological compiler — a system that maps antigen inputs to antibody outputs in a predictable, programmable way. This is a grand, almost sci-fi vision. It’s also one that nobody has pulled off before. It’s entirely possible that the biology is too noisy, or the immune system too complex, to make this work the way Popvax hopes. If that’s the case, this entire approach could turn out to be an expensive dead end.
For India more broadly, capital remains a serious constraint. We have only a handful of funds willing to back biotech, and most of them are still cautious, slow-moving, or waiting for someone else to go first. While the cost of launching a biotech company has come down thanks to cheaper machines and service models, there’s still a minimum threshold — and it’s higher than SaaS at the early stages. We need the big pharma players to put more money into R&D, not just compliance and manufacturing. And we need our venture capitalists to stop sitting on the sidelines. Yes, biotech is risky, binary bet. Yes, companies in this space can take many years to mature. But that’s true of venture capital as a whole; the difference now is that the entry price to do bio has dropped. So there’s no excuse not to try.
Another pressing challenge for Indian biotech is regulation. If India wants to compete with China or the US, we need a regulatory system that moves faster, with more clarity, and with fewer dead ends. Biotech innovation can’t be stuck waiting months for trial approvals or vague committee decisions. Just look at China: a big part of their biotech boom was triggered by top-down reform of their regulatory frameworks. India needs to follow suit with a predictable, founder-aligned, and globally credible regulatory regime.
Soham recently wrote about some of these challenges and opportunities in his essay Overcoming India’s Technological Cowardice. As he himself acknowledges, there’s a lot of work to be done on this point.
Acknowledging these obstacles doesn’t take away from the optimism. It grounds it. These are not reasons to give up; they’re the very problems that ambitious people come to solve. The optimism is real not because it ignores these risks, but because it stares them in the face and keeps building anyway.
VIII. ✨ YOU CAN JUST DO THINGS
The next decade belongs to biology, and biology belongs to all of us.
While the world remains captivated by artificial intelligence and digital frontiers, the most profound revolution of our time will unfold not in silicon, but in the carbon of our cells.
For millennia, the diseases that claim us, the aging that limits us, and the genetic lottery that shapes us have seemed like immutable laws of nature. But they are not laws at all. They are engineering challenges that have simply been waiting for the right tools, the right knowledge, and the right moment in history. That moment is now.
Cancer is corrupted cellular programming. Alzheimer's may be misfolded protein architecture. Even aging itself may be nothing more than accumulated errors in our biological operating systems — errors that can be identified, isolated, and corrected. What once seemed like the domain of gods is revealing itself to be the work of remarkably sophisticated, yet ultimately comprehensible, molecular machines.
This is why biology will define the coming era: not because it's trendy, but because it represents the final frontier of human engineering. We have digitized information, automated manufacturing, and optimized logistics. Biology is what remains — the most complex, most consequential, and most personal challenge we have left to master. When we do, we won't just build better products or faster computers. We will rebuild the very foundations of human experience.
What makes this moment so extraordinary is not just the scope of the opportunity, but its sudden accessibility. The towering barriers that once confined biological innovation to elite institutions and pharmaceutical giants are crumbling with breathtaking speed. DNA synthesis that once cost millions now costs pennies. Computational models that once required supercomputers now run on laptops. Machine learning systems can design new proteins from scratch, predicting their behaviour with near-perfect accuracy.
The story of Soham Sankaran illuminates this transformation. Here was a computer scientist with no formal training in biology, no connections in pharmaceuticals, and no institutional backing. Yet armed with nothing more than conviction, curiosity, and an internet connection, he taught himself molecular biology from first principles. Today, his vaccines are funded by the U.S. government and will be tested in humans by the National Institutes of Health. His journey from starting a robotics PhD to becoming generally-acknowledged as an Indian vaccine pioneer took less than five years.
This is not a fairy tale. It is a blueprint for what becomes possible when knowledge democratizes and barriers collapse. Soham's story is remarkable not because it's unique, but because it's becoming replicable.
The implications extend far beyond any single company or individual. For founders, this represents an unprecedented opportunity to tackle humanity's most pressing challenges without requiring decades of specialized training or billion-dollar budgets. For investors, it offers the chance to fund ventures that don't just generate returns, but fundamentally alter the human condition. For students and young professionals, it presents a field where impact scales with imagination rather than credentials.
The elephant of Indian biotech is stirring, testing the chains that have bound it for too long. The next chapter of our innovation story will not be written in software code alone, but in the language of life itself — in the elegant syntax of amino acids, the logic of cellular pathways, and the poetry of biological systems learning to heal themselves.
This is your invitation to join that story. Learn something new. Build something meaningful. Risk something significant. The tools are within reach, the knowledge is accessible, and the impact is limitless.
The future is biological. And biology, as it turns out, has been waiting for you all along.
—
Thanks for reading Tigerfeathers. If this moved you, share it with someone who needs to hear it. If you're building something in biotech, we want to learn from you. And if you're just beginning to explore this frontier — there has never been a better time to start.
Also – PopVax is hiring.
Acknowledgements
Thank you to Soham Sankaran, the PopVax team, and the team at TCG Life Sciences for all their help with this piece. Also for letting me cosplay as a biology researcher.
The contract details for PopVax’s $15M+ deal with Balvi state that the company must use the funds to develop an open-source COVID-19 vaccine upon which IP protections will not be enforced. However, the terms of this contract allow PopVax to own the core platform IP and use it for other (non-COVID) vaccines and therapeutics where it can enforce IP.
As for the $1M Gates Foundation contracts, they state that PopVax may use the funds to develop novel mRNA platform technologies where PopVax owns and can enforce the IP, with the caveat that the Gates Foundation has the right to use the technology or sublicense it to others under certain special circumstances to advance public health goals, as spelled out in their global access policy.
You might reasonably wonder — why would we ever want to manufacture an entire human genome or create synthetic organisms from scratch? The answer is that, in the long run, the ability to write life as easily as we read it could open up profound new frontiers: from designing organisms that produce novel medicines or clean up environmental waste, to building entirely new cellular chassis for research, bio-manufacturing, or even disease-resistant human cells. We’re still far from that, but the direction of travel is clear.
In the meantime, tools like CRISPR and base editing have already given us astonishing precision over existing genomes. CRISPR acts like molecular scissors — it allows scientists to cut a specific section of DNA inside a living cell and replace it with a new sequence. Base editing goes a step further: it can convert one DNA base into another without cutting the DNA at all. This makes it especially useful for correcting point mutations — single-letter typos in our genetic code that can have devastating consequences.
One remarkable example is the case of a baby in Philadelphia named KJ, who was born with a rare genetic condition that prevented his liver from breaking down ammonia. Thanks to base editing, doctors were able to correct the faulty gene directly in his cells. The edits worked, the ammonia cleared, and KJ got to go home.
Food and Drug Administration
You may remember stem cells as these shapeshifters that can turn into any kind of cell. In the early 1990s and early 2000s, there was a lot of excitement about the potential of stem cells to help us tackle several ailments. The only catch was that pluripotent stem cells — meaning the shapeshifter kind —could only be harvested from 5-7 day old human embryos in a process that would destroy them. As you can imagine, religious institutions and several civil society groups were less than enthused about the idea of factory farming human babies for the sake of science. So all research with pluripotent stem cells came to a halt.
Then, in 2006, a Japanese scientist called Shinya Yamanaka figured out a way to turn regular skin or blood cells into pluripotent stem cells. We now use this technology to grow organoids in labs, run tests on them which are super realistic and personalized to the patient, and then eventually use them in research, therapies, and transplants (like Eyestem does). We could also in theory turn these pluripotent stem cells into egg and sperm cells, paving the way for children fathered by two men or two women. For instance, quadruplets born of me and Rahul.







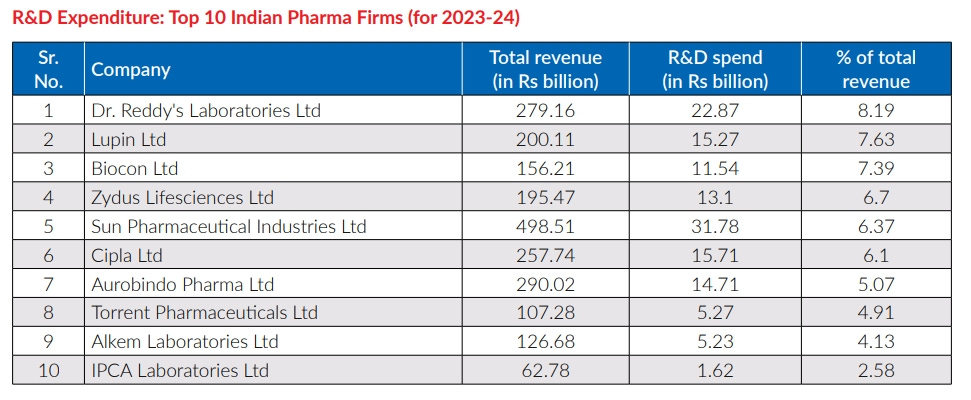




















Weekend read ready, looking forward to read after such a long wait.
Dam. This is in the top 0.001% of writing from India—fluid yet layered.
If biotech had a Netflix original documentary, this would be the script.